

Cosmetic
Dermatology in
Pursuit of Perfection
Epione Beverly Hills provides a complete
suite of fully-adaptable treatments to match
your unique goals, beauty, and style.
suite of fully-adaptable treatments to match
your unique goals, beauty, and style.
Dr. Simon Ourian
As the most sought-after A-list aesthetic
specialist in the world, Dr. Ourian has crafted
some of the most memorable cosmetic
aesthetics of modern times.
specialist in the world, Dr. Ourian has crafted
some of the most memorable cosmetic
aesthetics of modern times.




Cosmetic
Dermatology in
Pursuit of Perfection
Epione Beverly Hills provides a complete
suite of fully-adaptable treatments to match
your unique goals, beauty, and style.
suite of fully-adaptable treatments to match
your unique goals, beauty, and style.
Dr. Simon Ourian
As the most sought-after A-list aesthetic
specialist in the world, Dr. Ourian has crafted
some of the most memorable cosmetic
aesthetics of modern times.
specialist in the world, Dr. Ourian has crafted
some of the most memorable cosmetic
aesthetics of modern times.



Cosmetic
Dermatology in
Pursuit of Perfection
Epione Beverly Hills provides a complete suite of fully-adaptable treatments to match your unique goals, beauty, and style.







Dr. Simon Ourian
As the most sought-after A-list aesthetic specialist in the world, Dr. Ourian has crafted
some of the most memorable cosmetic aesthetics of modern times.


Aesthetics
and beauty
together
We perform every treatment with precision and care to achieve the results you want. Achieve stunning results through proven treatments matched to your unique beauty.
A-List Medical Professionals
Safety is at the core of Epione Beverly Hills. With a team of fully-trained professionals practicing in a state-of-the-art facility, we provide a safe, comfortable, and ideal experience.
Tailored Treatments
We believe in committing ourselves to every single treatment, adapting it in unique ways to match your individual goals and beauty. With an in-depth consultation process, we can build your treatment from the ground up for a luxury, tailored approach.
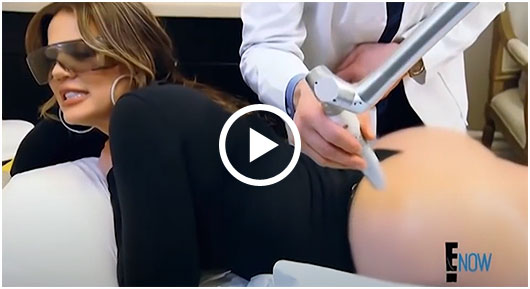
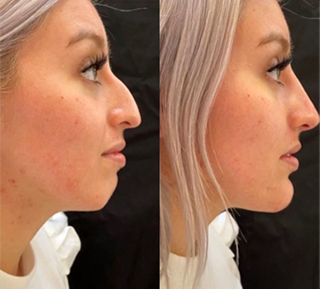
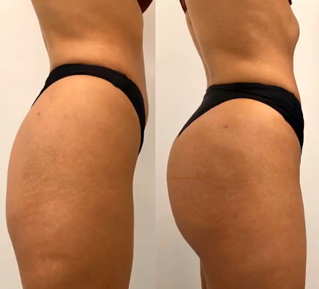
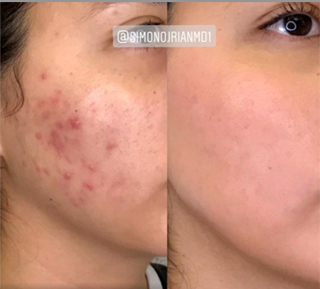
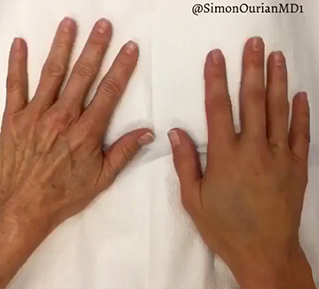
Sneak A Peek
Images often speak louder than words, and results speak for themselves. View our full gallery of before-and-after photos to see the changes possible through our full suite of treatments.
Aesthetics & Beauty Together
We perform every treatment with precision and care to achieve the results you want. Achieve stunning results through proven treatments matched to your unique beauty.
A-List Medical Professionals
Safety is at the core of Epione Beverly Hills. With a team of fully-trained professionals practicing in a state-of-the-art facility, we provide a safe, comfortable, and ideal experience.
Personalized Treatments
We believe in committing ourselves to every single treatment, adapting it in unique ways to match your individual goals and beauty. With an in-depth consultation process, we can build your treatment from the ground up for a luxury, tailored approach.
See Our Impact
Images often speak louder than words, and results speak for themselves. View our full gallery of before-and-after photos to see the changes possible through our full suite of treatments.
Ready to
Get Started?
Get Started?





